Chemical engineers at Monash University have developed an industrial process to produce acetic acid that uses the excess carbon dioxide(CO2) in the atmosphere, and has a potential to create negative carbon emissions.
Acetic acid is an important chemical used in several industrial processes and is an ingredient in household vinegar, vinyl paints and some glues. Worldwide industrial demand for acetic acid is estimated to be 6.5 million tonnes per year.
This world-first research, published in Nature Communications, shows that acetic acid can be made from captured CO2 using an economical solid catalyst to replace the liquid rhodium or iridium based catalysts currently used.
Liquid catalysts require additional separation and purification processes. Using a solid catalyst made from a production method that doesn’t require further processing also reduces emissions.
Lead researcher Associate Professor Akshat Tanksale said the research could be a widely adopted practice for industry.
“CO2 is over abundant in the atmosphere, and the main cause of global warming and climate change. Even if we stopped all the industrial emissions today, we would continue to see negative impacts of global warming for at least a thousand years as nature slowly balances the excess CO2,” Associate Professor Tanksale said.
“There is an urgent need to actively remove CO2 from the atmosphere and convert it into products that do not release the captured CO2 back into the atmosphere. Our team is focussed on creating a novel industrially relevant method, which can be applied at the large scale required to encourage negative emissions.”
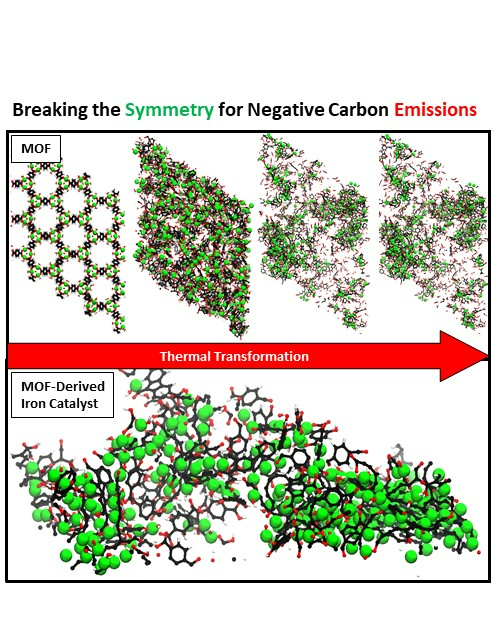
The research team first created a class of material called the metal organic framework (MOF) which is a highly crystalline substance made of repeating units of iron atoms connected with organic bridges.
They then heated the MOF in a controlled environment to break those bridges, allowing iron atoms to come together and form particles of a few nanometers in size (a nanometer is a billionth of a metre).
These iron nanoparticles are embedded in a porous carbon layer, making them highly active while remaining stable in the harsh reaction conditions. This is the first time an iron based catalyst has been reported for making acetic acid.
From an industrial point of view, the new process will be more efficient and cost effective. From an environmental perspective, the research offers an opportunity to significantly improve current manufacturing processes that pollute the environment.
This means a solution to slow down or potentially reverse climate change while providing economic benefits to the industry from the sales of acetic acid products.
The researchers are currently in the process of developing the process for commercialisation in collaboration with their industry partners as part of the Australian Research Council (ARC) Research Hub for Carbon Utilisation and Recycling.
The research project was supported by Monash University’s Engineering Researcher Accelerator Award, an ARC Discovery Project grant and collaborators from Hokkaido University and Pennsylvania State University.
Lead researcher Associate Professor Akshat Tankale is available for interviews.
About the image and video:
This shows a simulation of the thermal transformation of metal organic framework (MOF) which has symmetrical repeating units of metal (iron) atoms (green circles) linked by organic bridges (black and red). As we heat the MOF in a controlled environment, we see the bridges being broken and the iron atoms assimilate to make iron nanoparticles surrounded by leftover organic material. The final material is the catalyst which is used for converting CO2 into acetic acid.
Read the full research paper.
Watch simulation video of thermal transformation
- ENDS -
Media Enquiries
Loretta Wylde
Monash University
Media and Communications
T: +61 (0) 432 123 106
For any other topics on which you may be seeking expert comment, please contact the Monash University Media Unit on +613 9903 4840 or media@monash.edu For more Monash media stories, visit our news and events site.