Highlights
- Selection of biomarkers panel for Cleo’s ovarian cancer test-kit has been finalised
- Antibody development has advanced increasing confidence for commercial assay development and upscaling for commercial manufacturing
- The Company is in final stages evaluating four commercial antibody manufacturing partners
MELBOURNE, AUSTRALIA, 9 October, 2023: Ovarian cancer diagnostics company, Cleo Diagnostics Limited (ASX:COV) (CLEO, or the Company) confirms the delivery of early milestones in its development program targeting the initial surgical triage test market for its simple and accurate blood test.
TEST-KIT BIOMARKERS PANEL FINALISED
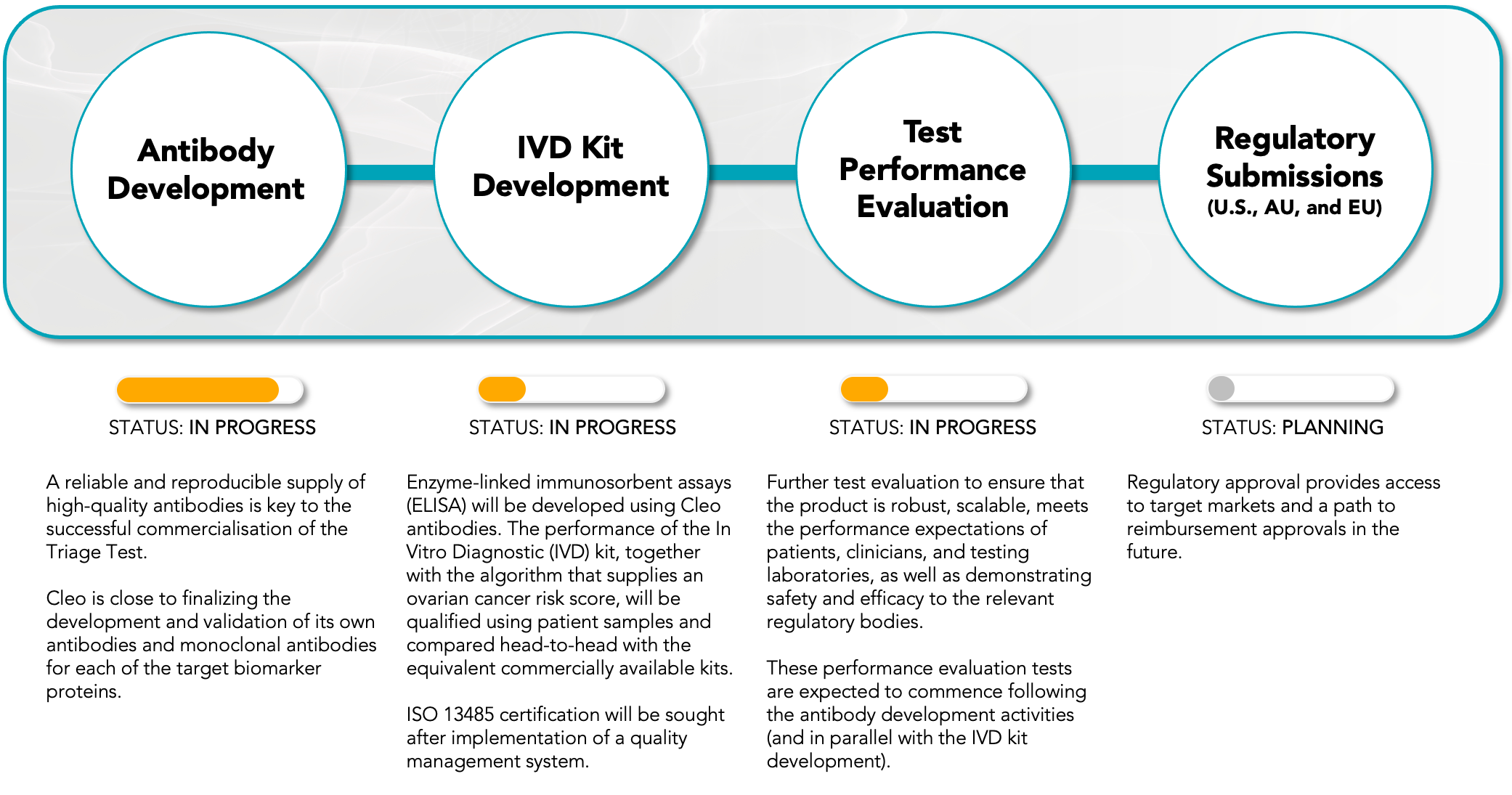
Cleo has finalised the selection of biomarkers to be used in its ovarian cancer test-kit, along with completing the development for a prototype of the proprietary scoring algorithm. The performance metrics of the test were evaluated in a clinical study of 334 patients, the results of which are being prepared for publication in a peer-reviewed medical journal. The Company expects the publication outcome to be reported to the market by the end of CY2023. The data cannot be released prior to publication due to the nature of the peer-review process. Concurrently, Cleo is also preparing a further patent application based on the findings.
ANTIBODY DEVELOPMENT
A key objective for the Company is to develop its own antibodies and target proteins which will allow control of supply, quality, cost and high-performance of key reagents that will underpin the consistent and reliable manufacture of test-kits. Cleo can confirm that Surface Plasmon Resonance Analysis has shown that the core antibodies of the CXCL10 active ratio test are binding to their respective targets with high affinity and are suitable for commercial assay development and upscaling in commercial manufacturing. Hybridomas to produce the supporting biomarker antibodies are also well progressed, with expected completion of the full test-kit panel in Q2 CY2024.
Commenting on the early development progress, CLEO Chief Executive, Richard Allman, said:
“The commercial foundation for our ovarian cancer test-kit targeting the initial surgical triage market is coming together quickly.
We are running a number of initiatives in parallel which are designed to place the Company in a strong position to achieve key milestones set this financial year.”
SELECTION PROCESS FOR ANTIBODY MANUFACTURING PARTNER
Cleo is in the late stages of evaluating four commercial antibody manufacturing partners as part of a robust tender process. The evaluation process considers a competitive review of the capabilities of each potential partner to ensure that the partner ultimately selected can deliver commercial product to the standard required by Cleo, which is largely set by regulatory bodies such as the Food and Drug Administration (FDA) and potential customer groups.
Figure 1: Indicative high-level timeline for the commercial development of Cleo’s ovarian cancer test-kit for the initial Surgical Triage Test market.
For more information, or media inquiries, contact:
Elvis Jurcevic
Investor Relations
+61408 268 271
ej@cleodx.com
or
Richard Allman
Chief Executive Officer
+613 9614 0000
office@cleodx.com