Highlights
- Results from the first clinical validation study of the CleoDX Triage Test, performed in a 334 patient cohort, have been published in the peer-reviewed journal “Cancers”
- The article, entitled ‘A novel predictive multi-marker test for the pre-surgical identification of ovarian cancer’ provides a detailed overview of the high performance of Cleo’s ovarian cancer diagnostics test
- The article concluded that Cleo’s ovarian cancer test:
• Was highly accurate with 95% sensitivity1 / 95% specificity2;
• Correctly discriminated malignant from benign samples; and
• Out-performed and was superior to current clinical methods. - Peer-review provides important validation of Cleo’s technology and commercial strategy, targeting the surgical triage market where accurate and sensitive identification of malignant tumours is essential.
MELBOURNE, AUSTRALIA, 6 November, 2023: Ovarian cancer diagnostics company, Cleo Diagnostics Limited (ASX:COV) (CLEO, or the Company) is pleased to announce the publication of an article on its triage test for ovarian cancer.
PEER REVIEWED PUBLICATION
Cleo’s first clinical validation study for its ovarian cancer triage test has been published in the peer-reviewed international journal ‘Cancers’. The results confirm the high accuracy of the CleoDX Triage Test, which was independent of menopausal status, and showed that it out-performed the two most widely used clinical scoring systems (the “Risk of Malignancy Index” and “Risk of Malignancy Algorithm”) for discriminating benign from malignant ovarian disease.
A copy of the publication is available here: https://www.mdpi.com/2072-6694/15/21/5267
Moreover, the CleoDX surgical triage test correctly identified 81% of early-stage cancer patients in the cohort.
Commenting on the publication, Cleo Chief Scientific Officer, Dr Andrew Stephens, said:
“These results confirm that our core technology is robust and accurate, and most importantly can identify cancers at an early stage. These results strongly support our planned further development of this core technology aimed at ovarian cancer screening in the longer term”.
CLEO Chief Executive, Richard Allman, added:
“This is an important step forward as we work towards our goal of an FDA approved triage test. I look forward to providing further updates to the market as we progress through our development program.”
The next step in development will confirm functionality of the commercially available kits in an independent clinical trial, the results of which will be submitted to the FDA for regulatory approval. Cleo anticipates commencement of this trial before the end of CY2023.
OPPORTUNITY FOR CLEO AND OVARIAN CANCER DIAGNOSTICS MARKET
At present there is no clinically routine pre-surgical method for reliable evaluation and differentiation of benign vs malignant ovarian cancer tumours. Radical surgery is the cornerstone of cancer management, with complete hysterectomy being the norm. Removal of the ovaries, however, predisposes women to multiple co-morbidities including increased risk of cardiovascular disease, dementia and certain cancers amongst others. There is a clear need to differentiate benign vs malignant cases pre-surgically to enhance patient outcomes.
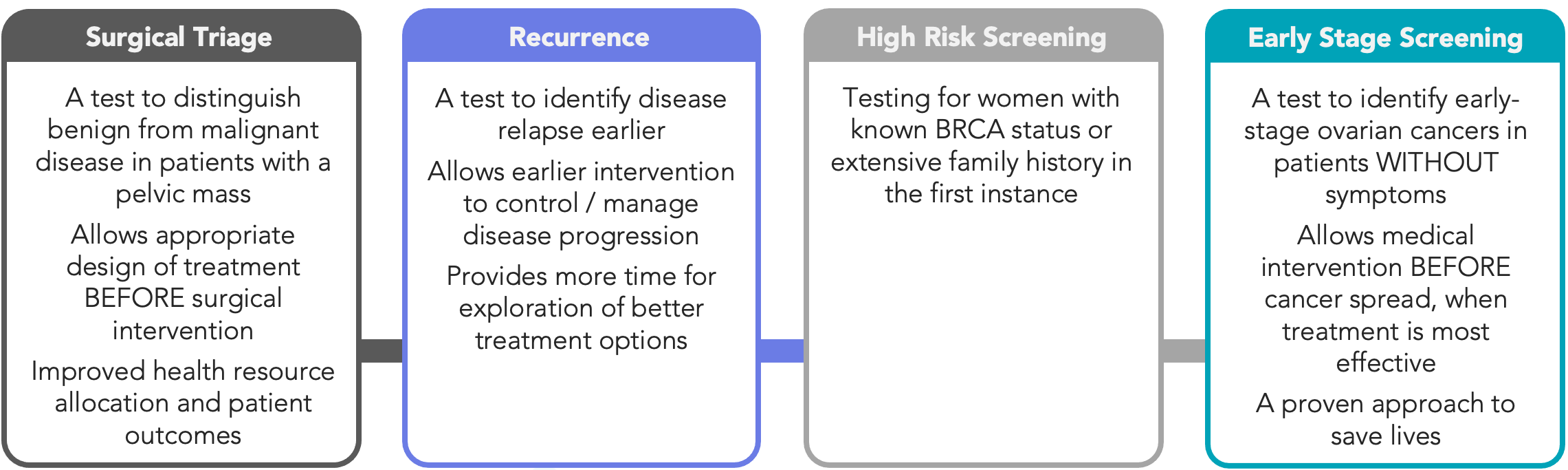
Cleo has defined a staged execution strategy to deliver it’s simple blood test which is focused on three key markets across pre-surgical triage testing, high-risk/recurrence detection, and broader screening programs. Achieving a positive outcome here from a peer-reviewed publication, has a material impact on the Company’s pathway with respect to the initial triage market. The Company will now use the publication of its test performance to further define the scope of the triage market.
Figure 1: Ovarian cancer diagnostic markets targeted by Cleo
For media inquiries, please contact:
Elvis Jurcevic
Investor Relations
+614 08 268 271
ej@cleodx.com
1 Sensitivity refers to the ability of a test to correctly identify patients with the tested for disease (true positive rate).
2 Specificity refers to the ability of a test to correctly identify people without the tested for disease (true negative rate).