VILNIUS, LITHUANIA / ACCESSWIRE / November 16, 2023 / Northway Group is embarking on a project to establish Europe's largest biotechnology hub, BIO CITY, in Vilnius, the capital of Lithuania. It includes 6 large biotechnological complexes - 4 state-of-the-art GMP manufacturing plants and 2 advanced scientific research centres - that will be built in an area equivalent to 10 football fields. The total investment for this biotech campus is projected to reach approximately 7 billion euros over the next decade.
Northway Group is in the process of constructing Europe's largest biotechnology hub, with the inaugural Gene Therapy Center set to be operational in 2024.
"A science-based economy, supported by bright minds and intelligent entrepreneurs, is the foundation for Lithuania's long-term economic prosperity. In the past, our growth was constrained by a lack of fossil resources, but today, we are boldly moving forward, relying on modern technologies. The new biotechnology hub embodies the direction of Lithuania's innovative economy. It also promises new inventions that will enable people with serious illnesses to become full members of society, thereby reducing exclusion," says the President of the Republic of Lithuania, Gitanas Nausėda.
Prof. Vladas Algirdas Bumelis, founder and CEO of Northway Biotech and Celltechna, key components of the Northway Group, highlighted Lithuania's strong global standing in biotechnology. The aim of the BIO CITY project is to further solidify this position with four advanced biomanufacturing facilities and two innovative research centres, significantly boosting Lithuania's prominence in the international biotech sphere.
The Speaker of the Seimas, parliament of Lithuania, states that the new biotech city being developed in Vilnius will strengthen the competitiveness of our country. "Lithuanian life sciences industry has ambitions and potential to become a global leader in this field: a leader who will significantly contribute to the development of scientific research for the well-being of man, nature and planet, and will facilitate new opportunities to deal with global health, sustainable development and other challenges," says Viktorija Čmilytė-Nielsen.
Vision of BIO CITY: A European Biotechnology Leader
"We envision BIO CITY as a frontrunner in the European biotechnology, by uniquely integrating various biotech segments into a single, synergistic ecosystem. This multifunctional complex will catalyse interdisciplinary collaborations, the quick realisation of ideas and technological advancements. Our unique model, which brings together diverse biotechnology fields in one location, is set to revolutionise the European biotech landscape," said Prof. V. A. Bumelis.
Gene Therapy Centre will Open in 2024
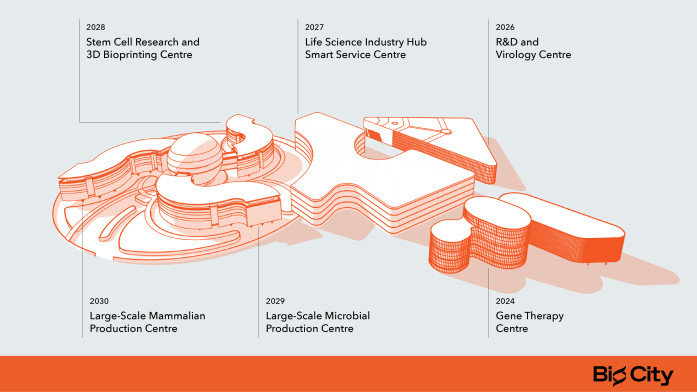
The first facility to open its doors in the biotech hub BIO CITY will be the Gene Therapy Centre, which is currently under construction and is being built by Northway Group's subsidiary, Celltechna. This centre, the first and so far the only one of its kind in the Baltic States, will bolster Lithuania's role in gene therapy, addressing the needs of the 280 million individuals worldwide who are affected by genetic diseases.
"Our state-of-the-art facility will be instrumental in both research and production, offering new treatments for previously incurable diseases. This will not only augment our CDMO (Contract Development and Manufacturing Organisation) capabilities, but also position us for global competition and collaborations," added Prof. V. A. Bumelis.
The Gene Therapy Centre, which is expected to become operational in the second quarter of 2024, will specialise in gene therapy research and GMP manufacturing. Representing an investment of 50 million euros, the facility will span 8,000 square metres and is anticipated to create over 100 high-value jobs. The centre will work in synergy with Northway Biotech. Established in 2004, Northway Biotech is a leading provider of CDMO services in the field of biologics, with a focus on the development and manufacturing of recombinant proteins and antibodies.
A Comprehensive Lithuanian Biotech Hub
By 2030, BIO CITY will see the inauguration of five additional complexes, including centres for R&D and Virology, Life Sciences Industry Smart Services, Stem Cell Research and 3D Bioprinting, as well as two large-scale production centres for mammalian and microbial products. The entire BIO CITY complex will span an area equivalent to 10 football fields, with the total investment expected to reach around 7 billion euros over the next decade.
"We will not only focus on contract development and manufacturing services, but will also invest significantly in the operation of scientific research centres. Scientific activity enhances a country's competitiveness and generates value in various forms, beyond just the economic aspect. Modern biotechnologies, such as gene editing and cell therapies, are advancing rapidly. Lithuania can pride itself on having some of the most talented scientists and robust expertise in these areas. The development of the biotech campus in Vilnius means we are poised to foster new partnerships with innovative startups, research institutions and pharmaceutical companies on a global scale. We are actively seeking partnerships and offer a warm invitation to investors who are enthusiastic about joining this exciting venture," said Prof. V. A. Bumelis.
Upon its completion, BIO CITY is expected to offer employment to approximately 2,100 highly skilled professionals, including scientists, biotechnologists, and medical engineers.
Lithuania is Among the Leaders in the Global Biotechnology Market
The global biotechnology market, currently valued at over 1,130 billion euros, is anticipated to grow to be worth more than 2,775 billion euros by 2030. Lithuania holds a strong position in this market, ranking among the Top 35 innovative countries in the biotechnology field, according to Scientific American Worldview.
"Every year, Lithuania is mentioned in the field of Life Sciences more often, and the ambitious BIO CITY project will contribute to our leadership. Our vision is coming to life - we are talking about world-class Life Sciences infrastructure and a competitive sector capable of building innovative products. In 2022, companies in the sector posted combined revenues of 1.5 billion euros, while exporting their goods to more than 100 countries. Overall, Life Sciences is a leading sector in Lithuania, when it comes to creating and implementing innovative solutions," states Aušrinė Armonaitė, the Minister of the Economy and Innovation.
Over 80 life science companies operate in Lithuania, contributing about 2.5% to the country's GDP. The Northway group, a key player in Lithuania's biotech sector, manages seven companies: five in Lithuania and one each in the UK and the US, with the US entity being recognised as the largest biotech investor from the Baltic region in recent years. Employing more than 200 specialists, these companies provide services to a diverse array of international biopharmaceutical firms, ranging from small to large enterprises, predominantly operating in both Europe and the US.
BIO CITY Contacts:
Vladas Algirdas Bumelis
CEO and Chairman of the Board
[email protected]
Contact Information:
Vladas Bumelis
CEO and Chairman of the Board
[email protected]
Related Files
BIO CITY LOGO_ORANGE.png
BIO CITY LOGO_BLACK.png
Related Images


SOURCE: Northway Healthcare Group

View source version on accesswire.com:
https://www.accesswire.com/804258/the-largest-biotech-city-in-europe-will-soon-be-built-with-an-investment-amounting-to-7-billion-euros
