HIGHLIGHTS
- FY24 glaucoma segment sales up 37% to US$15.3 million compared to pcp
- FY24 USA Sales up 73% to US$11.4 million compared to pcp
- 2H FY24 USA Sales up 77% compared to pcp
- Fourth consecutive half-year of revenue growth in the USA
- Additional sales specialists recruited in the USA, materially improving geographic coverage
- Material improvement in glaucoma segment operating result in H2FY24 expected
- Last quarter sales demonstrate an annualised revenue run rate of approximately US$18 million (A$27.7 million)(1) (2)
Nova Eye Medical Limited (ASX: EYE) (Nova Eye Medical or the Company) is pleased to provide an update on glaucoma segment sales for the year ended 30 June 2024. This update is based on unaudited financial statements.
Group glaucoma segment revenue for the year ended 30 June 2024 is expected to be US$15.3 million (A$23.5 million), up 37% on the previous corresponding period (pcp) (1). This result includes annual sales in the USA of US$11.4 million, up 73% on FY23.
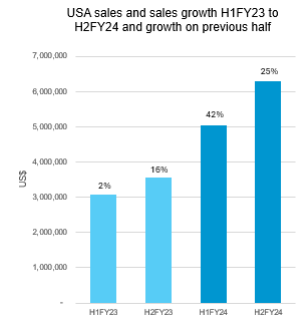
Improved sales growth in the USA has now been demonstrated via sales growth over four consecutive half-year periods.
This strong performance was underpinned by the recruitment of additional sales specialists in the USA and continued investments in clinical data collection and peer-to-peer marketing programs.
With the Company prioritising investment in sales growth in the USA during the period, sales revenue in markets outside the USA remained in line with PCP.
In FY25 the Company will pursue sales growth in strategically important markets outside of the USA, including in Western Europe and Asia.
The sales growth achieved in the second half of FY24 is expected to drive a material improvement in glaucoma segment operating result in the second half of FY24 compared with both the first half of FY24 and the full year FY23.
Further details are provided in the presentation also released to the ASX.
The Company expects to release its audited financial statements in late August 2024.
(1) Based on average FY24 exchange rate of USD0.65 = AUD1.00 (2) Based on the last 3 months of FY24, revenue annualised
Link to ASX Release: Record Sales Result in FY24
Link to ASX Release: Presentation - Glaucoma Segment Sales Update for the year ended 30 June 2024
For additional information about Nova Eye Medical and its technologies, please visit: www.nova-eye.com
Key Facts:
- FY24 glaucoma segment sales up 37% to US$15.3 million compared to pcp
- FY24 USA Sales up 73% to US$11.4 million compared to pcp
- 2H FY24 USA Sales up 77% compared to pcp
- Fourth consecutive half-year of revenue growth in the USA
- Additional sales specialists recruited in the USA, materially improving geographic coverage
- Material improvement in glaucoma segment operating result in H2FY24 expected
- Last quarter sales demonstrate an annualised revenue run rate of approximately US$18 million (A$27.7 million)
About us:
Nova Eye Medical Limited is a medical technology company that develops, manufactures and sells a portfolio of proprietary ophthalmic treatment technologies and devices. Used by eye surgeons globally, these technologies include iTrack™ Advance a minimally invasive consumable glaucoma surgical device that restores the eye’s natural outflow pathway to lower pressure inside the eye and to eliminate patient reliance on anti-glaucoma medications for mild-moderate glaucoma. The Molteno3® glaucoma drainage device platform is designed to enhance surgical utility and optimize clinical outcomes for long-term IOP control in cases of severe glaucoma. It also offers the benefit of a simplified and faster surgical procedure. With its sales headquarters based in Fremont, California, Nova Eye Medical is supported by a global network of distribution partners. Manufacturing facilities are located in Fremont, California and Dunedin, New Zealand.
Contact details:
Company
Tom Spurling
Managing Director
+61 417 818 658
tspurling@nova-eye.com
Company
Kate Hunt
Chief Commercial Officer
+61 404 080 679
khunt@nova-eye.com
Investors
Mark Flynn
Investor Relations
+61 416 068 733
mflynn@nova-eye.com