Triastek announces that its 3D printed drug product D23 (Budesonide Ileum Targeted Tablets for the treatment of IgAN) has achieved positive results in recent clinical study. D23 is budesonide delayed-release tablet made by 3D printing using Melt Extrusion Deposition (MED®) process first developed in scale by Triastek based on its proprietary 3D Microstructure for Intestine Targeting (3DμS®-IT) platform. This proprietary platform allows for precise intestinal targeting of drug release and delivery ensuring that the budesonide reaches the ileal area of the intestine where the disease originates and budesonide would be most beneficial. It is anticipated that this targeted delivery will provide significant advantages in the treatment of patients with IgAN disease over current therapies.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20250216386739/en/
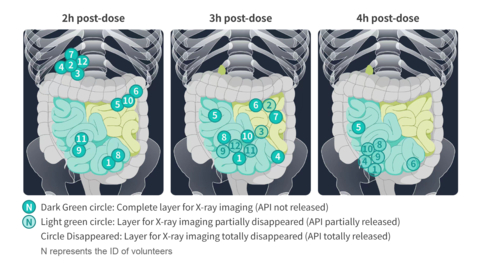
X-ray imaging results of D23 (Budesonide Ileum Targeted Tablet) (Graphic: Business Wire)
The D23 clinical study was randomized, open-label, single-dose, 2-sequence, 4-period, fully repeated crossover trial design. Using an innovative method labeled D23 tablet components and observing in vivo tablet GI transit via X-ray imaging, the study examined the time-course of D23 GI transit and corresponding budesonide pharmacokinetics.
The X-ray imaging results demonstrated that budesonide from D23 tablets is not released until the tablets reach the ileum providing maximum budesonide exposure at the desired site of action. Since the site of disease origin is in the ileum, this targeted delivery is hypothesized to optimize budesonide effects on the immune response and treat IgAN at the site of disease origin. The budesonide pharmacokinetic (PK) profile after D23 administration was correlated with the X-ray results, corresponding to budesonide delivery at the ileum in a consistent and predictable manner.
Following on these positive results, D23 is advancing to the next phase of clinical trials to evaluate the clinical effectiveness of targeted delivery of budesonide with IgAN patients via D23 printed tablets.
The MED® 3D printing process and 3D microstructure designs allow for precise control of the release behavior of drugs in the body by the choice of delay layer material, layer thickness and the resulting composition to degrees not previously possible, in contrast to traditional tableting techniques utilizing a delay layer and drug core. In addition to creating a delay, the subsequent drug release can also be accomplished in various manners, including immediate, sustained and pulsed release.
View source version on businesswire.com: https://www.businesswire.com/news/home/20250216386739/en/
Contact details:
Public Relation, Jiaxin Tao, [email protected]

