
-
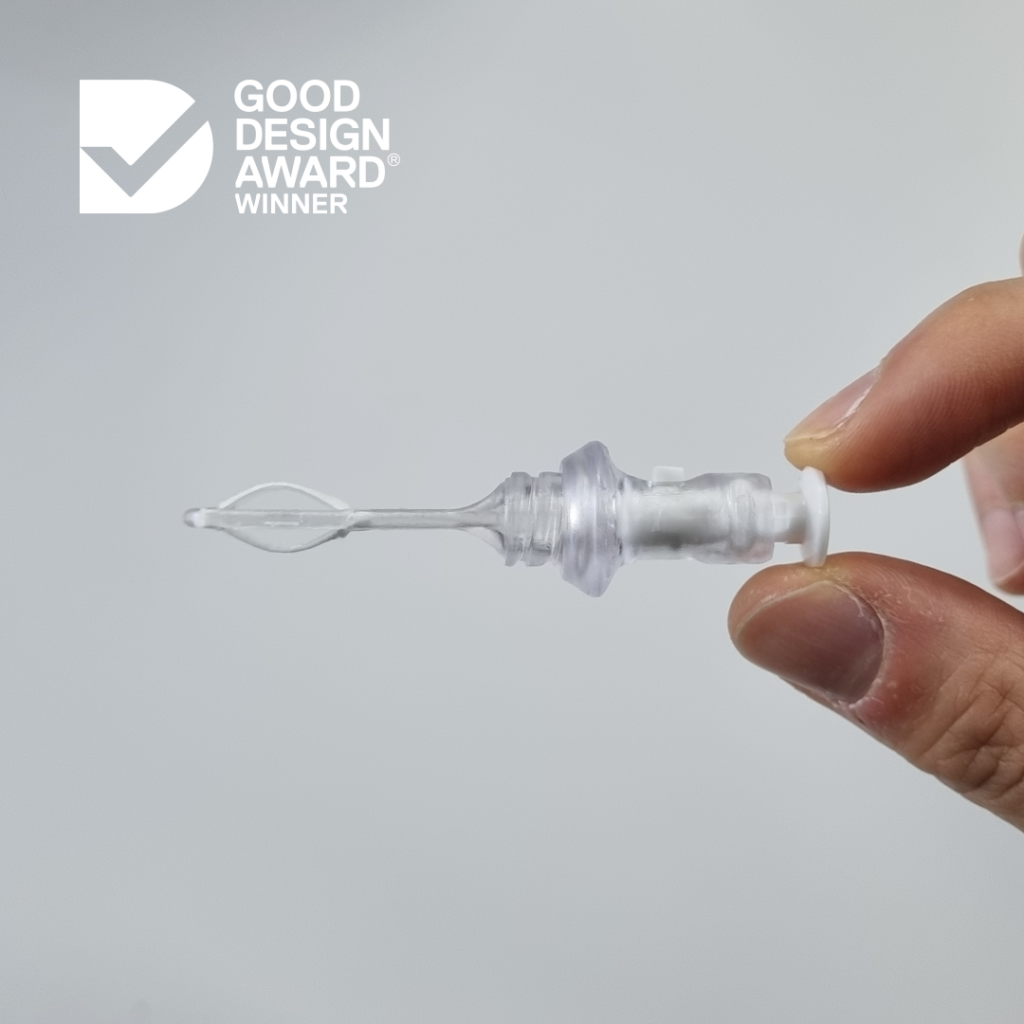
Diag-Nose.io's ABEL Microsampler® wins Australian Good Design Award in Product Design category
-
Praised by judges as "setting new benchmark for good design in the Med Tech space"
-
Device enables precision nasal fluid sampling without requiring specialised training or complex equipment
-
Innovation helps advance diagnosis and management of airway disease, lung cancer and broad variety of other applications through reliable data collection
MELBOURNE, Australia, 14th October 2025 – Diag-Nose.io is proud to announce that its nasal liquid biopsy device, the ABEL Microsampler® has been recognised with a prestigious Australian Good Design Award Winner accolade in the Product Design category. The Australian Good Design Awards is one of the world’s longest-running international design programs, celebrating excellence in design and innovation across disciplines.
Jonathan Limpah, Senior Engineer at Diag-Nose.io, shared the team’s excitement about the award and what it means for the future of respiratory research and clinical care “We designed the ABEL Microsampler® to simplify precision nasal sampling. It requires no specialised training or complex equipment, just reliable data to better understand and treat respiratory disease. We’re honoured to have our work recognised with a Good Design Award, and it’s inspiring to see design-led innovation making a real impact in healthcare.”
Aligned with this year’s theme, “Rewarding, Defining and Celebrating Better _______.” the ABEL Microsampler® demonstrates how thoughtful design can transform complex science into simple, human-centred solutions. Recent studies highlight the urgent need for standardised and accessible nasal sampling methods that enable research ranging from vaccine development [1, 2] to understanding how environmental factors influence respiratory health [3]. The ABEL Microsampler® enables precision nasal fluid sampling that is easy to use and accessible, helping researchers and clinicians collect reliable, high-quality data to advance the diagnosis and management of airway disease, lung cancer, and a broad variety of other applications.
The Good Design Awards Jury commented “The ABEL Microsampler® is a novel nasal liquid biopsy device that enables reproducible, site specific sampling for diagnostics and drug discovery. A great example of professional Industrial Design and production engineering being brought together to solve a complex medical problem. Overall, this is very clever design that enhances respiratory diagnostic sample integrity and user comfort. This project truly sets a new benchmark for good design in the Med Tech space. Well done.”
1. Bladh, O., et al., Comparison of SARS-CoV-2 spike-specific IgA and IgG in nasal secretions, saliva and serum. Frontiers in Immunology, 2024. Volume 15 - 2024.
2. Zhang, X., et al., Exploring the standardized detection and sampling methods of human nasal SARS-CoV-2 RBD IgA. Frontiers in Immunology, 2025. Volume 16 - 2025.
3. Mirmozaffari, Y., et al., Cytokine Sampling in the Nasal Cavity and Paranasal Sinuses. Ear, Nose & Throat Journal, 2025. 0(0): p. 01455613251346570.
-ENDS-
About us:
About Diag-Nose.io
Diag-Nose.io, founded in 2020 as a spin-out of the Stanford Biodesign ENT Innovation program, is a biotechnology company focused on translating the complexities of the unified airway into precision diagnostic and drug discovery solutions. Their precision medicine technology combines advanced proteomics, computational biology, and AI (machine learning) to create a scalable respiratory biology model. This innovation aims to help clinicians prescribe the right treatments faster and enable researchers to accelerate the development of new therapies.
For further information, visit www.diag-nose.io
Contact details:
For interviews or enquiries in relation to this release, contact:
Nicole Papoutsis
0422 418 099
[email protected]


