Highlights
• University of Miami (UMiami) commences clinical use of CT:VQ™
• UMiami becomes second U.S. academic medical centre (AMC) after Stanford to begin clinical use of CT:VQ™, validating early commercialisation strategy
• Arrangement provides three months of introductory pricing prior to full commercial terms
• Lahey Hospital & Medical Center signs two-year agreement for IQ-UIP™
Melbourne, Australia, 10 December 2025: 4DMedical Limited (ASX: 4DX, “4DMedical” or the “Company”), a global leader in respiratory imaging technology, today announces that the University of Miami has entered into a commercial arrangement for the clinical use of CT:VQ™. 4DMedical also announces a new agreement with Lahey Hospital & Medical Center for IQ-UIP™.
CT:VQ™ commercial launch at UMiami
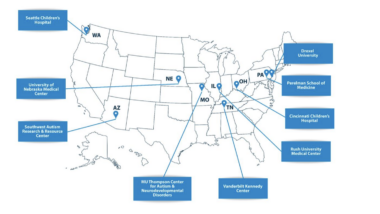
The University of Miami, a leading academic medical centre with a nationally recognised pulmonary medicine program, has commenced clinical use of CT:VQ™ under a structured launch framework. An introductory pricing period will apply for the first three months prior to full commercial terms, supporting early clinical adoption and workflow establishment.
4DMedical is working to build a strong network of leading AMCs to drive broad adoption of CT:VQ™ across the U.S. market. UMiami is the second U.S. academic medical center, following Stanford, to adopt CT:VQ™ for clinical use in the past two months, providing strong validation of the Company’s commercialisation strategy.
CT:VQ™ at RSNA
The commercial launch at UMiami follows exceptionally strong clinician engagement at RSNA, with leading radiologists from across the United States engaging with 4DMedical to learn more about CT:VQ™, and how it can help both their practices and their patients.
As part of joint marketing efforts at RSNA, Philips and 4DMedical held a breakfast event that was very well attended by senior radiologists from AMCs and the VA. The breakfast featured an expert panel that shared real-world insights on workflows, clinical evidence, and implementation strategies for integrating CT:VQ™ into screening, triage, patient management and therapy planning. The discussion shone a spotlight on how functional lung maps are driving sharper clinical decisions across radiology and pulmonology, advancing health system goals for improved access, quality, and efficiency in cardiopulmonary care. A full video of the breakfast event can be found here.
Lahey Hospital & Medical Center agreement
4DMedical has signed a two-year agreement with Lahey Hospital & Medical Center (Lahey Clinic), a nationally recognised leader in pulmonary medicine and interventional pulmonology. Under the agreement, Lahey will deploy 4DMedical's Lung Density Analysis™ (LDA) and IQ-UIP™ software on an annual subscription model with capacity for up to 24,000 scans per annum. IQ-UIP™ provides quantitative CT analysis for the assessment of idiopathic pulmonary fibrosis and related interstitial lung diseases.
4DMedical MD/CEO and Founder Andreas Fouras said:
I am very pleased to see CT:VQ™ deployed at the University of Miami. The immediate transition from strong RSNA engagement to commercial operations demonstrates the significant momentum we are seeing from clinicians seeking a contrast-free, high-resolution alternative to nuclear medicine VQ scans.
This commercial launch comes on the heels of incredibly strong clinician interest at RSNA. I had the chance to meet leading radiologists from all over the U.S. who were eager to connect with 4DMedical, dive into the technology, and explore how it can make a real difference for their practices and their patients. It was energising to see just how excited they were about what we’re building.
4DMedical is moving with incredible speed. I am excited by the momentum and look forward to keeping you updated as we advance our strategy of winning key U.S. AMCs as reference sites for CT:VQ™.
To read the full announcement, please click here
About us:
About 4DMedical
4DMedical Limited (ASX:4DX) is a global medical technology company revolutionizing respiratory care withadvanced imaging and artificial intelligence. Its patented XV Technology® transforms standard scans into rich, functional insights that allow physicians to detect, diagnose, and monitor lung disease earlier and with greater precision.
4DMedical’s expanding software portfolio includes the FDA-cleared XV Lung Ventilation Analysis Software (XV LVAS®), CT LVAS™, and the ground-breaking CT:VQ™ solution designed to set new benchmarks in cardiothoracic imaging by combining ventilation and perfusion analysis.
Delivered seamlessly through a Software-as-a-Service (SaaS) model, 4DMedical’s solutions integrate into existing hospital infrastructure, enhancing physician productivity and enabling more personalized patient care. With the addition of advanced AI capabilities from its 2023 acquisition of Imbio, 4DMedical continues to push the boundaries of medical imaging to redefine how respiratory disease is understood and treated worldwide.
Learn more at www.4dmedical.com
Contact details:
For further information, please contact:
Media Enquiries
[email protected]
Corporate
Investor Relations
[email protected]
Julia Maguire
[email protected]