Highlights
• UC San Diego Health, consistently ranked in the top 10 in the U.S. for Pulmonology & Lung Surgery, commences clinical use of CT:VQ™
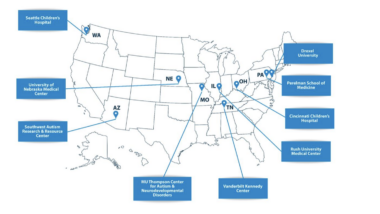
• UC San Diego Health becomes the fourth U.S. academic medical center to adopt CT:VQ™, following Stanford, University of Miami and Cleveland Clinic
• Four academic medical center deployments achieved within four months of FDA clearance, validating 4DMedical’s commercialisation strategy
• Arrangement provides introductory pricing through to March 2026 prior to full commercial terms
Melbourne, Australia, 7 January 2026: 4DMedical Limited (ASX: 4DX, “4DMedical” or the “Company”), a global leader in respiratory imaging technology, today announces that UC San Diego Health has entered into a commercial arrangement for the clinical use of CT:VQ™.
CT:VQ™ commercial launch at UC San Diego Health
UC San Diego Health (UCSD) is one of the nation's leading academic health systems and has consistently ranked in the top 10 in the U.S. for Pulmonology & Lung Surgery. UCSD has commenced clinical use of CT:VQ™ under a structured launch framework whereby introductory pricing will apply through March 31, supporting early clinical adoption and workflow establishment, before transitioning to full commercial terms.
Led by Dr. Jonathan Chung, Chief of the Division of Cardiothoracic Imaging and Professor of Radiology, UCSD's cardiothoracic imaging program is internationally renowned for pioneering advanced imaging techniques and clinical innovation. The addition of CT:VQ™ to UCSD's imaging portfolio positions the institution at the forefront of functional lung imaging, providing a contrast-free, high-resolution alternative to traditional nuclear medicine VQ scans.
UCSD joins Stanford University, University of Miami, and Cleveland Clinic as the fourth U.S. academic medical centre (AMC) to deploy CT:VQ™ for clinical use. This expanding network of leading AMCs powers 4DMedical's strategic approach of establishing reference sites at the nation's most prestigious institutions, creating a powerful foundation for broader market adoption.
Building momentum across leading AMCs
The commercial launch at UCSD represents continued validation of 4DMedical's commercialisation strategy. In just over four months since FDA clearance in August 2025, 4DMedical has secured four of America's most respected academic medical centres, each recognised globally for clinical excellence and imaging innovation.
These deployments demonstrate the compelling clinical value proposition of CT:VQ™: eliminating the need for radioisotope and contrast administration, providing superior image resolution compared to nuclear medicine, seamlessly integrating into existing CT imaging workflows, and enabling access to reimbursement pathways that support sustainable clinical adoption.
As opinion leaders in cardiothoracic imaging, these institutions serve as critical reference sites, accelerating physician education, clinical validation, and confidence in CT:VQ™ as the new standard of care for ventilation-perfusion imaging.
Dr. Jonathan Chung, Chief of Cardiothoracic Imaging at UC San Diego Health, said:
"Radiologists are experts of anatomic imaging, but as our understanding of lung disease expands, purely structural assessment limits our diagnostic reach. CT:VQ™ now enables functional ventilation and perfusion evaluation layered on top of traditional inspiratory and expiratory CT. This represents a significant leap forward and will allow radiologists to make diagnoses with more sensitivity and specificity."
4DMedical MD/CEO and Founder Andreas Fouras said:
"UCSD is consistently ranked in the top 10 for pulmonology & lung surgery and is home to a world-class cardiothoracic imaging program. Their adoption of CT:VQ™ represents another powerful validation of our technology and our strategic approach to commercialisation.
In just over four months since FDA clearance, we've established CT:VQ™ at four of America's leading academic medical centres: Stanford, University of Miami, Cleveland Clinic, and now UCSD. This rapid adoption by elite institutions demonstrates both the transformative potential of CT:VQ™ and the strength of our go-to-market execution.
These prestigious AMCs serve as powerful anchors for our commercialisation strategy. Combined with our Philips partnership and growing commercial pipeline, we are building unstoppable momentum as we establish CT:VQ™ as the new standard of care in pulmonary imaging.
The momentum continues to accelerate, and I look forward to sharing further progress with shareholders as we advance through 2026."
To read the full announcement, please click here
About us:
About 4DMedical
4DMedical Limited (ASX:4DX) is a global medical technology company revolutionizing respiratory care withadvanced imaging and artificial intelligence. Its patented XV Technology® transforms standard scans into rich, functional insights that allow physicians to detect, diagnose, and monitor lung disease earlier and with greater precision.
4DMedical’s expanding software portfolio includes the FDA-cleared XV Lung Ventilation Analysis Software (XV LVAS®), CT LVAS™, and the ground-breaking CT:VQ™ solution designed to set new benchmarks in cardiothoracic imaging by combining ventilation and perfusion analysis.
Delivered seamlessly through a Software-as-a-Service (SaaS) model, 4DMedical’s solutions integrate into existing hospital infrastructure, enhancing physician productivity and enabling more personalized patient care. With the addition of advanced AI capabilities from its 2023 acquisition of Imbio, 4DMedical continues to push the boundaries of medical imaging to redefine how respiratory disease is understood and treated worldwide.
Learn more at www.4dmedical.com
Contact details:
For further information, please contact:
Media Enquiries
[email protected]
Corporate
Investor Relations
[email protected]
Julia Maguire
[email protected]