- FoLix™, the first FDA-cleared fractional non-ablative laser for hair loss, has won Best New Dermatology Technology Award 2025 and NewBeauty Award for Best Laser Treatment 2025
- The treatment is expanding into Australia following successful US launch in June 2024, offering a non-invasive solution for hair loss that requires only 4-6 monthly sessions
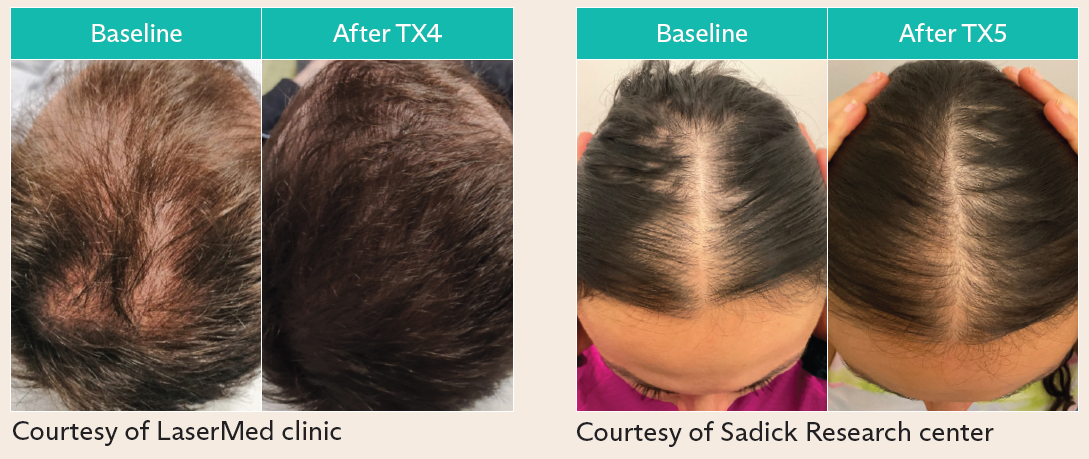
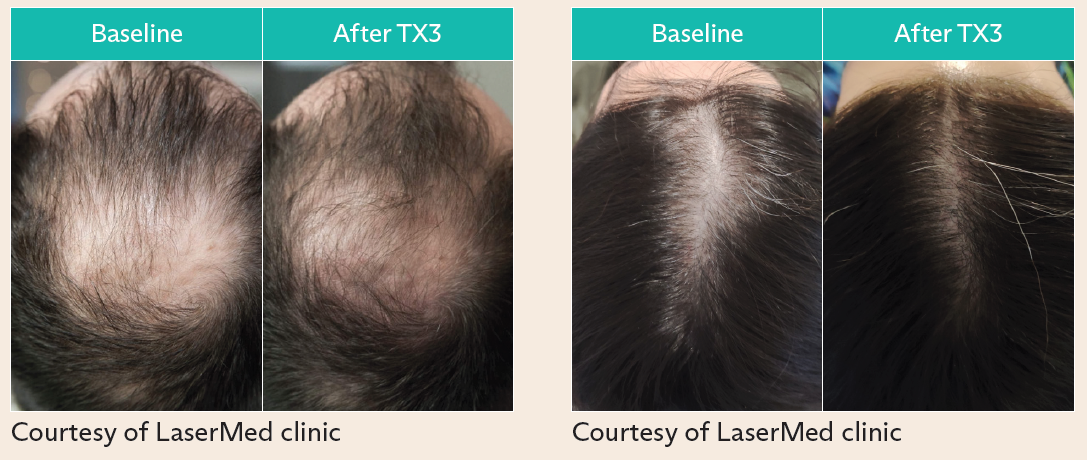
- FoLix™ uses proprietary laser technology to stimulate hair follicles, enhance blood flow and promote tissue regeneration without chemicals, needles or surgery
- The treatment addresses a widespread concern, as over 85% of men and 50% of women experience some form of hair loss during their lifetime
- Developed by Lumenis, a global leader in energy-based medical devices, FoLix™ is suitable for adults with Fitzpatrick skin types I to IV seeking non-pharmaceutical hair loss treatment
|
|
|
Sydney, Australia, January 29, 2026 — Lumenis BE ANZ Pty Ltd - A leading energy-based medical device company, is proud to announce the expansion of FoLix™ into Australia - the first and only FDA-cleared fractional non-ablative laser for hair loss.
Following its success in the United States since its debut in June 2024, Lumenis is marking a significant milestone in its international expansion with the launch of FoLix™ into the Australian market to help both men and women to look and feel their best by offering an effective and safe hair loss solution.
FoLix’s novel technology harnesses non-ablative and non-invasive fractional laser, and proprietary technology tailored for scalp hair to stimulate hair follicles. FoLix™ delivers precise pulses of laser that leverage the body’s natural healing processes to stimulate hair follicles, enhance blood flow and promote tissue regeneration. FoLix™ can produce results in only four to six monthly sessions with no chemicals, needles, anesthesia, surgery or downtime. Expanding availability of FoLix™ is a meaningful step in improving patient care, as more than 85% of men and 50% of women experience some form of hair loss in their lifetime, which can deeply affect self-esteem and overall quality of life.
“With FoLix™, we’re not just offering a treatment, we’re empowering people to reclaim their confidence and feel like their best selves,” said Tzipi Ozer-Armon, CEO of Lumenis.
“The expansion into Australia marks a pivotal milestone in our mission to transform the hair loss space with a solution that is science-backed, safe, and clinically proven. This momentum, reinforced by our NewBeauty and MEDTECH award wins, fuels our commitment to accelerate access to FoLix™ worldwide.”
FoLix’s ability to easily fit into a wide range of practice groups’ preexisting offerings showcases how the treatment has achieved such rapid expansion into various international markets within its first year since launch. With patients’ growing interest in non-surgical solutions with minimal discomfort that fit easily into their lifestyle – FoLix™ stands out as the premier choice for hair loss treatment.
“This is an amazing treatment that, in my experience, is less painful than platelet-rich plasma treatments,” explains Maple Grove, MN dermatologist Ronda Farah, MD. “It gives patients an effective option for hair stimulation that doesn’t require ongoing medication.” Designed for comfort and efficacy, FoLix™ offers a high-tech approach for people seeking hair-loss treatment.
For more information about FoLix™ please visit lumenis.com.au/aesthetics/products/folix/
PR & Media Enquiries / To trial a FoLix™ treatment, please contact
Kate Boissett - Head of Marketing Lumenis Aesthetics
P 0422 734 621
E [email protected]
About FoLix™
FoLix™ is the first and only non-ablative, non-invasive fractional laser device indicated for improving the appearance of scalp hair in adult males and females with Fitzpatrick skin types I to IV, who are seeking treatment for hair loss without the need for pills or injections. Using fractional laser technology, FoLix™ works by stimulating hair follicles. It treats the scalp in a precise, grid-like pattern, delivering energy to boost circulation and encourage growth potential.
About the “Best New Dermatology Technology Solution of 2025” MEDTECH Breakthrough Award
FoLix™ - The winner of this competitive category due to the combination of scientific innovation, patient-centric design and measurable clinical impact. MedTech Breakthrough’s 9th annual Awards program celebrates excellence and innovation in the health and medical technology industry, recognizing the companies, products, and solutions driving meaningful progress and improving patient care. Spanning a wide range of categories, the awards highlight the groundbreaking work of Lumenis that is transforming the aesthetic and medical landscape.

About the “Best Laser Treatment for Hair Loss” NewBeauty’s 2025 Beauty Award
Highlighting the FoLix™ treatment’s effectiveness powered by its groundbreaking approach. NewBeauty’s 15th Annual Beauty Awards recognizes the very best beauty products and treatments, highlighting award-winning and cutting-edge technology solutions, presented by the aesthetic experts of NewBeauty.
Being recognized with such a prestigious award underscores the advantages of FoLix’s groundbreaking approach and demonstrates Lumenis’ dedication to developing novel, energy-based devices to address some of the most pressing aesthetic and medical related concerns.
About us:
Lumenis is a global leader in the medical aesthetic market and is a world-renowned expert in developing and commercializing innovative energy-based technologies, including Laser, Intense Pulsed Light (IPL) and Radiofrequency (RF). For more than 50 years, Lumenis’ ground-breaking products have redefined medical treatments and set technological and clinical gold-standards, revolutionizing existing treatment methods, and creating solutions for previously untreatable conditions. Lumenis is a portfolio company of EQT Private Capital Asia. For more information regarding Lumenis’ range of clinical solutions, please visit: https://lumenis.com.au
Contact details:
To trial a FoLix™ treatment, please contact
Kate Boissett - Head of Marketing - Lumenis Aesthetics Australia
P 0422 734 621
[email protected]