
Family First South Australian Legislative Council candidate Deepa Mathew says Premier Peter Malinauskas can no longer dismiss concerns about men in women’s prisons as a “niche issue” after a major backflip by the Victorian government.
Victorian Labor Premier Jacinta Allan has been dragged kicking and screaming into acknowledging the obvious danger posed by placing biological males in women’s prisons.
Authorities in Victoria have now placed accused child rapist Matthew ‘Kaleigh’ Green — who identifies as a woman — in a men’s prison after he was charged with 194 sex offences, including multiple counts of child rape.
“For months the Victorian government stonewalled questions about the risks of housing biological males in women’s prisons,” Ms Mathew said.
“Now when confronted with a biologically male prisoner accused of horrific sexual crimes, they have quietly admitted the blindingly obvious — that men pose a danger to women in prison.”
Ms Mathew said the development exposed the weakness of Mr Malinauskas’ dismissal of the issue in South Australia.
“When concerns were raised about men being housed in women’s prisons in South Australia, the Premier waved them away as a ‘niche issue’,” she said.
“That was an extraordinary thing to say given the controversy surrounding the women’s unit at Port Augusta Prison.”
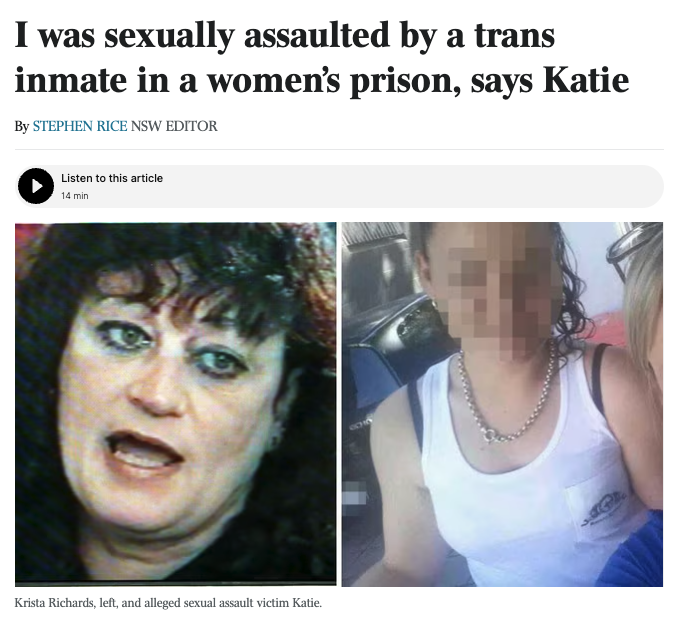
Last year serious allegations emerged that a female prisoner had been sexually assaulted by a biologically male inmate who identified as a woman and had been placed in the women’s facility.
The accused prisoner denied the allegation and charges were not laid, but the case sparked serious concerns from female inmates, prison staff and women’s advocates about the safety of housing biological males in women’s prisons.
“For the women inside Port Augusta Prison this is not a niche issue,” Ms Mathew said.
“Women in prison are among the most vulnerable people in our society and they should never be forced to share cells, showers or living quarters with biological males.”
Ms Mathew said Victoria’s decision showed that even Labor governments knew the risks.
“Jacinta Allan has been dragged kicking and screaming to this point after months of refusing to answer basic questions about women’s safety,” she said.
“Now there is nowhere for Peter Malinauskas to hide.”
“If Victoria can acknowledge the obvious, South Australia should do the same and guarantee that women’s prisons are reserved for women.”
Contact details:

