HIGHLIGHTS
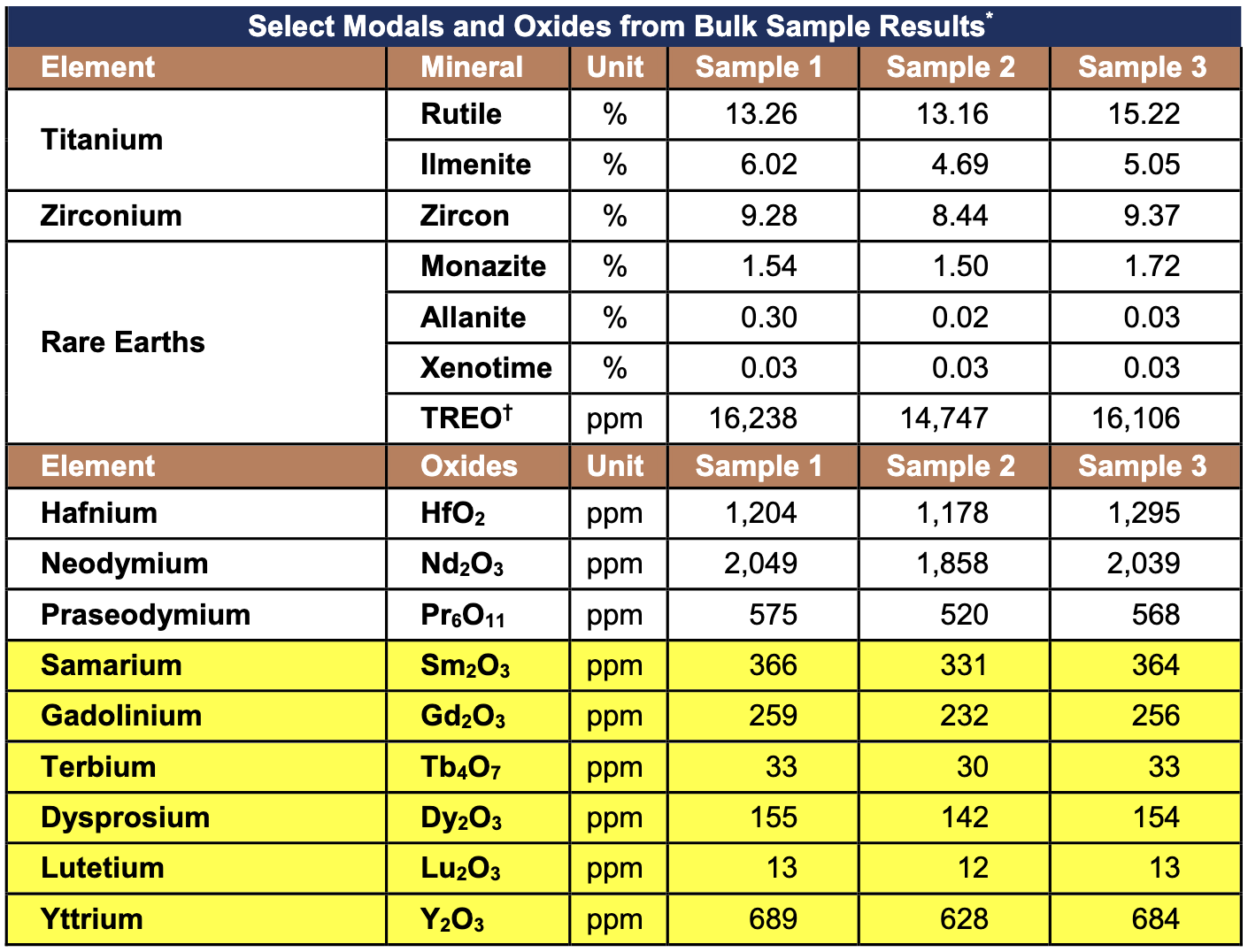
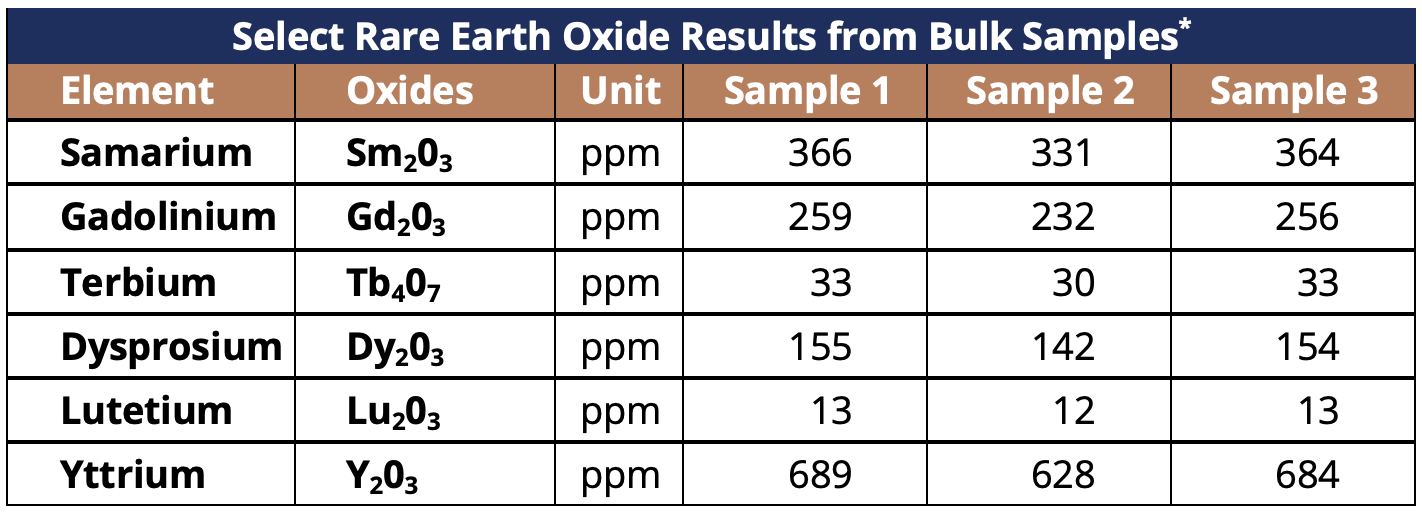
- Bulk sample results indicate Orion Project has relatively high-grades of six of seven rare earth oxides recently banned for export by the Chinese Government (highlighted below):
- Metallurgical test works are ongoing and include consideration of rare earth element mineral by-products
- Magnet rare earths and titanium metal are both strategic critical raw materials in the EU (two of 17)
- Light rare earths, heavy rare earths, titanium and hafnium are critical raw materials in the EU (four of 34)
- Iberian Critical Minerals Pty Ltd now has path to 95% interest in Orion Project after acquisition of half of remaining third party interest
- Spanish experienced geologist and mining executive Mr Lachlan Rutherford to join Board as Executive Director
- Focus remains on commencing drilling activities
______________________
Osmond Resources Limited (ASX: OSM) (Osmond or the Company) is pleased to confirm positive ongoing progress at the Orion EU Critical Minerals Project, located in southern Spain.
Ongoing metallurgical test works are planned to consider recoveries of rare earth oxides given the strategic nature of the oxides in the context of the recent ban on exports by the Chinese Government. Results from the 150kg bulk sample analysed by SGS in Canada suggest the Orion Project has relatively high-grades in six of seven medium and heavy rare earths, that are now subject to Chinese Government’s export bans.
The Company is pleased to announce it has agreed to allot 500k shares in the Company to support the acquisition by Iberian Critical Minerals Pty Ltd (ICM) of 50% of the remaining third-party interest in the Orion Project not controlled by ICM. The 50% equates to 5% of the shares in the holding company that owns a 100% interest in the Orion Project post completion of a Scoping Study. ICM is the parent company of the Spanish company that owns the shares in the holding company. The Company’s staged acquisition of ICM remains subject to permit award.
Osmond is also pleased to welcome Mr Lachlan Rutherford to the Board as an Executive Director. Mr Rutherford is a geologist with over 25 years broad experience including over 18 months as in-country manager for a Spanish tungsten project. Mr Rutherford’s appointment enables founding Chairman, Mr Rhod Grivas to step off the Board to allow him to focus on other opportunities.
Commenting on the progress, Osmond CEO and Managing Director, Anthony Hall, said:
“Whilst rutile and zircon remain our focus given the current pricing environment, we have not lost sight of the very strategic opportunity we have with rare earths. We have the potential to support the EU strategic critical minerals 2030 extraction goals with the potential to become the first EU based source of rare earths, including the critically important magnetic rare earths.
And these goals have become a lot more important given the Chinese Government’s recent ban on exports of seven medium and heavy rare earths.
Once we confirm continuity of our two primary high-grade seams, we will be pushing hard to fast-track development activities.”
Orion EU Critical Minerals Project
Overview
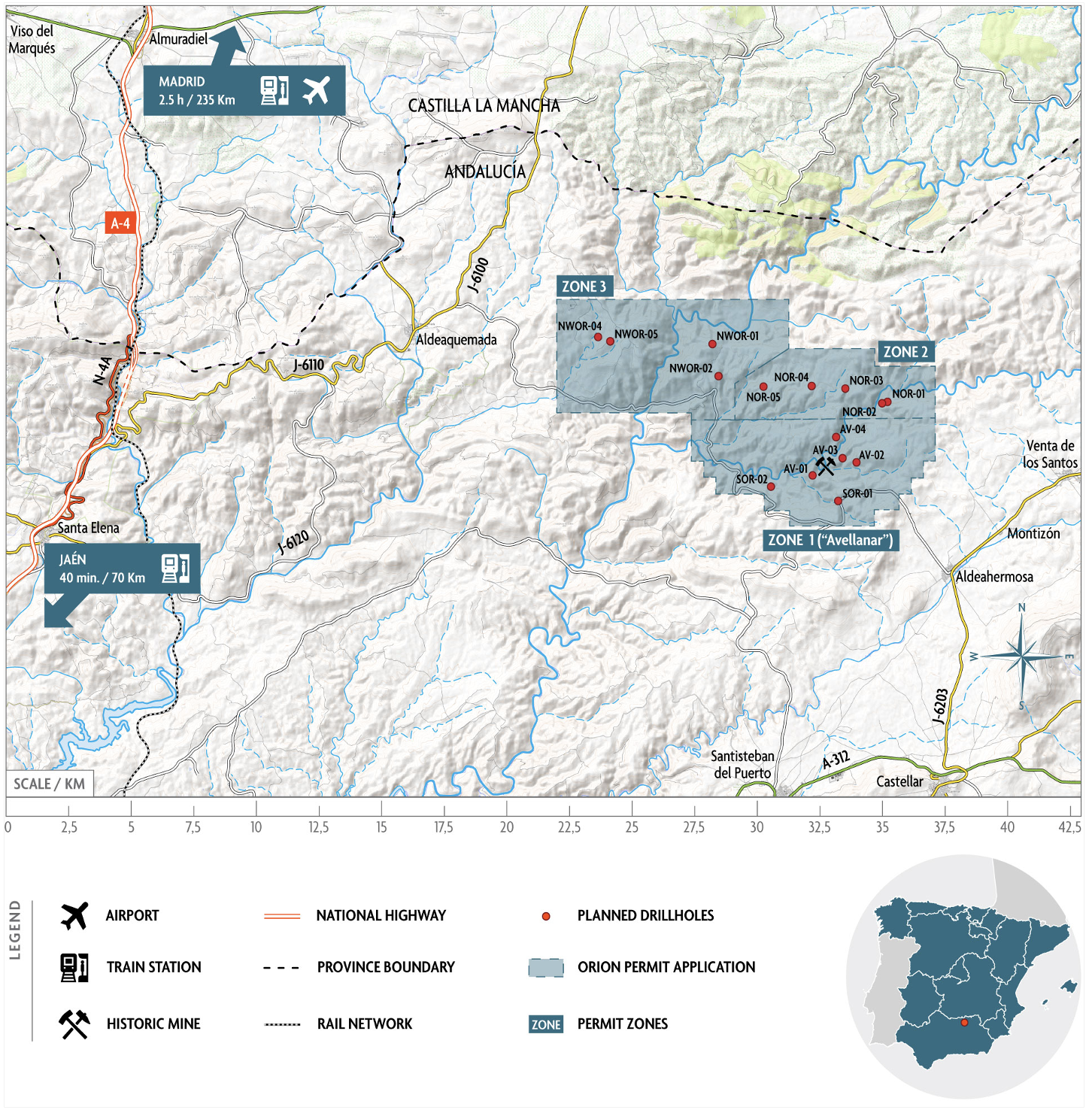
The Orion EU Critical Minerals Project (the Project) is located in Jaén Province, Andalucía, Southern Spain (refer Figure 1 below). The Project includes 288 Spanish mining units (cuadrículas mineras) covering an area of 86.4km2.
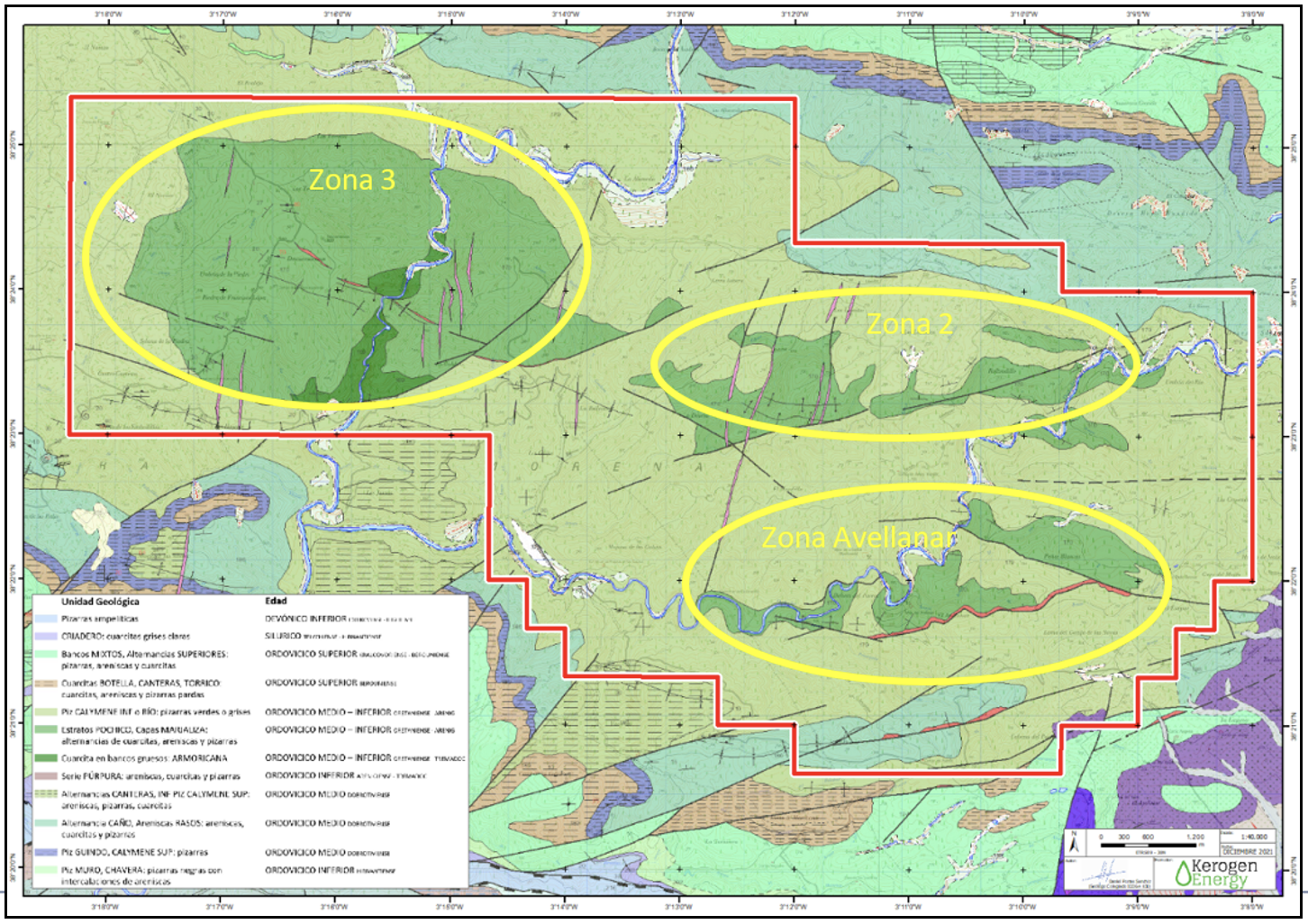
It is a siliciclastic geological system with various layers rich in critical minerals including rutile (titanium), zircon, hafnium, and rare earth elements. The Project area was explored for thorium and uranium in the 1950s and 1960s and includes a historic galena mine. Three initial target areas have been identified with an initial focus on the Avellanar Zone (Zone 1) (refer Figure 2 below).
Metallurgical Test Works
In anticipation of positive drilling results designed to confirm continuity of the two primary high-grade seams across the three target zones, the Company has been progressing metallurgical test works using material from the 150kg bulk sample sent to SGS in Canada.
These test works are designed to ensure the Company is able to fast track development activities, removing the flow sheet from the critical path to deliver a Scoping Study.
Given the strategic nature to the EU of light and heavy rare earths and considering the relatively high-grades, the Company intends to progress activities associated with confirming appropriate flow sheets to monetise rare earth credits. These test works are even more important given the recently announced export bans by the Chinese Government on seven rare earth oxides.
The Table below shows the relatively high-grade results from the bulk sample analysis across six of the seven rare earth oxides subject to the ban.
Table 1 – Table Showing Select Rare Earth Oxide Results from 160kg Bulk Sample
Acquisition of 50% of Remaining 10% Interest in Orion Project
On 22 April 2025, an agreement was signed in Spain for the Company to support the acquisition of 50% of the remaining third-party interest in the Orion Project via the acquisition of shares in Green Mineral Resources SL (GMR), the 100% owner of the Orion Project. ICM owns a 100% interest in Omnis Mineria SL that in turn has the right to now move to a 95% interest in GMR upon completion of a Scoping Study.
Once the Scoping Study is complete, the third-party holder of the final 5% interest can then elect to contribute on a pro rata basis or convert the 5% interest to a gross revenue royalty of 2.5% that can be bought back for US$750,000. The royalty commences after the first 1.2m tonnes of product from the mine has been sold.
Prior to the acquisition, the third-party vendors held 49% of the shares in Green Mineral Resources SL. Omnis Mineria SL now owns 75.5% of the shares until it moves to 95% on completion of a Scoping Study.
The Company has agreed to allot 500,000 ordinary shares to the third-party vendor in full consideration for the acquisition.
The Company’s staged acquisition of ICM remains subject to permit award.
Board Changes
Effective 23 April 2025, Mr Lachlan Rutherford has been appointed to the Board as an Executive Director.
Mr Rutherford is a geologist with over 20 years of commercial and exploration experience in industrial mineral, precious metal, and base metal projects. He has held positions in venture capital, public companies and stockbroking, focusing on business development, corporate strategy, project management and analytical roles. His international experience includes work on critical mineral projects in Spain and Finland.
Mr Rutherford holds a Doctorate of Philosophy, a Masters of Business Administration, and a Bachelor of Science with Honours. He is also a member of the Australian Institute of Mining and Metallurgy.
Mr Rutherford will receive A$15,000 per month (inclusive of superannuation) and a long-term incentive of 2,000,000 share options with a 75c strike and a term of four years from 23 April 2025. These options will be issued pursuant to ASX Listing Rule 10.12 (Exception 12) and do not require shareholder approval. Short term incentives will be at the discretion of the Board and within the Company’s existing remuneration guidelines. Either party may terminate the engagement by giving three (3) months written notice.
With the appointment of Mr Rutherford, the Company’s founding Chairman, Mr Rhod Grivas has elected to step off the Board to enable him to focus on other opportunities. Mr Grivas was instrumental in the Company’s successful IPO and the acquisition of the Company’s flagship Spanish projects. The Company is grateful for his contribution, and in particular, his stewardship of the Board through IPO to the current date.
-Ends-
Media and Investor contact:
Anthony Hall
Managing Director and CEO
[email protected]
+61 417 466 039
Elvis Jurcevic
Investor Relations
[email protected]
+61 408 268 271