
HIGHLIGHTS
- Record December quarter with global sales of US$6.1 million, up 38% on pcp and 25% on the September 2025 quarter
- Global six-month sales of US$10.9 million, up 29% on pcp
- Last Twelve Months (LTM) global sales of ~US$21 million (A$32.2 million), up 24% on prior twelve months and three times higher than the industry growth rate of ~8%(1)
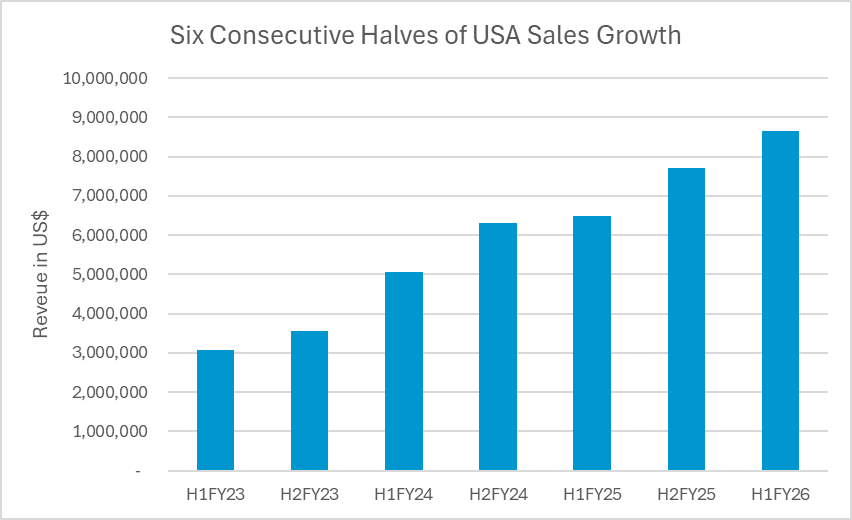
- Six consecutive halves of sales growth in the USA at a CAGR of ~40%
Nova Eye Medical Limited (ASX: EYE) (Nova Eye Medical or the Company) is pleased to report a record sales result for the December 2025 quarter and continued strong growth for the six months ended 31 December 2025. These results are subject to audit.
Sales Performance
The December quarter result of US$6.1 million reflects continued strong demand in the United States, supported by consistent growth in Rest of World markets. The iTrack technology has now been used in approximately 180,000 surgical cases worldwide. Its minimally invasive, rejuvenative method of action is becoming increasingly recognised by more surgeons as our commercial efforts continue. Sales growth in the twelve months to 31 December 2025 was 24% up on the prior year. This is three times the industry growth rate of ~8% estimated by Marketscope (1).
Regional Performance Summary (US$’000s)(2)
|
US$000's (unaudited) |
Q2FY25 (3 mths to Dec 24) |
Q2FY26 (3 mths to Dec 25) |
H1FY25 (6 mths to Dec 24) |
H1FY26 (6 mths to Dec 25) |
Growth on Qtr PCP |
Growth on Half PCP |
|
USA |
3,333 |
4,498 |
6,476 |
8,559 |
35% |
32% |
|
Germany |
512 |
503 |
870 |
877 |
-2% |
1% |
|
Direct |
3,845 |
5,001 |
7,346 |
9,436 |
30% |
28% |
|
ROW |
210 |
469 |
329 |
816 |
123% |
148% |
|
Sales (excl China) |
4,055 |
5,470 |
7,675 |
10,252 |
35% |
34% |
|
China |
350 |
603 |
710 |
603 |
72% |
-15% |
|
Group |
4,405 |
6,073 |
8,385 |
10,855 |
38% |
29% |
Sales results in China in H1FY26 do not include any sales of iTrack™ Advance, which was approved for sale in China in September 2025. Sales are expected to commence in 2026.
Last Twelve Months (LTM) Revenue
Group revenue for the twelve months ended 31 December 2025 reached ~US$21 million, representing 24% growth on the prior twelve-month period. Growth was led by the United States (USA) and Rest of World (ROW) markets.
|
LTM Dec 2024 (US$’000’s) |
LTM Dec 2025 (US$’000’s) (2) |
Growth |
LTM Dec 2025 (A$’000’s) (3) |
|
|
USA |
12,777 |
16,272 |
27% |
25,033 |
|
Germany |
1,720 |
1,835 |
7% |
2,823 |
|
Direct |
14,497 |
18,107 |
25% |
27,856 |
|
ROW |
1,020 |
1,788 |
75% |
2,750 |
|
Sales (excl China) |
15,516 |
19,895 |
28% |
30,606 |
|
China |
1,385 |
1,053 |
-24% |
1,620 |
|
Group |
16,901 |
20,948 |
24% |
32,226 |
USA sales growth summary
The six months to 31 December 2025 delivered the sixth consecutive half of sales growth since the launch of iTrack™ Advance in the USA. The compounded annualised growth rate across this period is ~40%.
Footnotes
- Marketscope Glaucoma Surgical Devices Report August 2025: 2024 to 2025 growth rate for “Tube shunts, Microstents, Subconjunctival shunts, Canaloplasty, Goniotomy”
- Based on management sales records, not audited
- Based on FX rate of A$1.00 = US$0.65
Read our ASX Announcement here: Record Sales Result for December 2025 Quarter
To learn more about our Company, visit: Nova Eye Medical
About us:
Nova Eye Medical Limited is a medical technology company that develops, manufactures and sells a portfolio of proprietary ophthalmic treatment technologies and devices. Used by eye surgeons globally, these technologies include iTrack™ Advance, a minimally invasive consumable glaucoma surgical device that restores the eye’s natural outflow pathway to lower pressure inside the eye and to eliminate patient reliance on anti-glaucoma medications for mild-moderate glaucoma. The Molteno3® glaucoma drainage device platform is designed to enhance surgical utility and optimize clinical outcomes for long-term IOP control in cases of severe glaucoma. It also offers the benefit of a simplified and faster surgical procedure. With its sales headquarters based in Fremont, California, Nova Eye Medical is supported by a global network of distribution partners. Manufacturing facilities are located in Fremont, California and Dunedin, New Zealand.
For additional information about Nova Eye Medical and its technologies, please visit: www.nova-eye.com
Contact details:
Company
Tom Spurling
Managing Director
+61 417 818 658
Investors
Mark Flynn
Investor Relations
+61 416 068 733